BrainAce
In vivo brain tumor diagnostics by adaptive computational lensless fiber endoscopy
Medical Need |
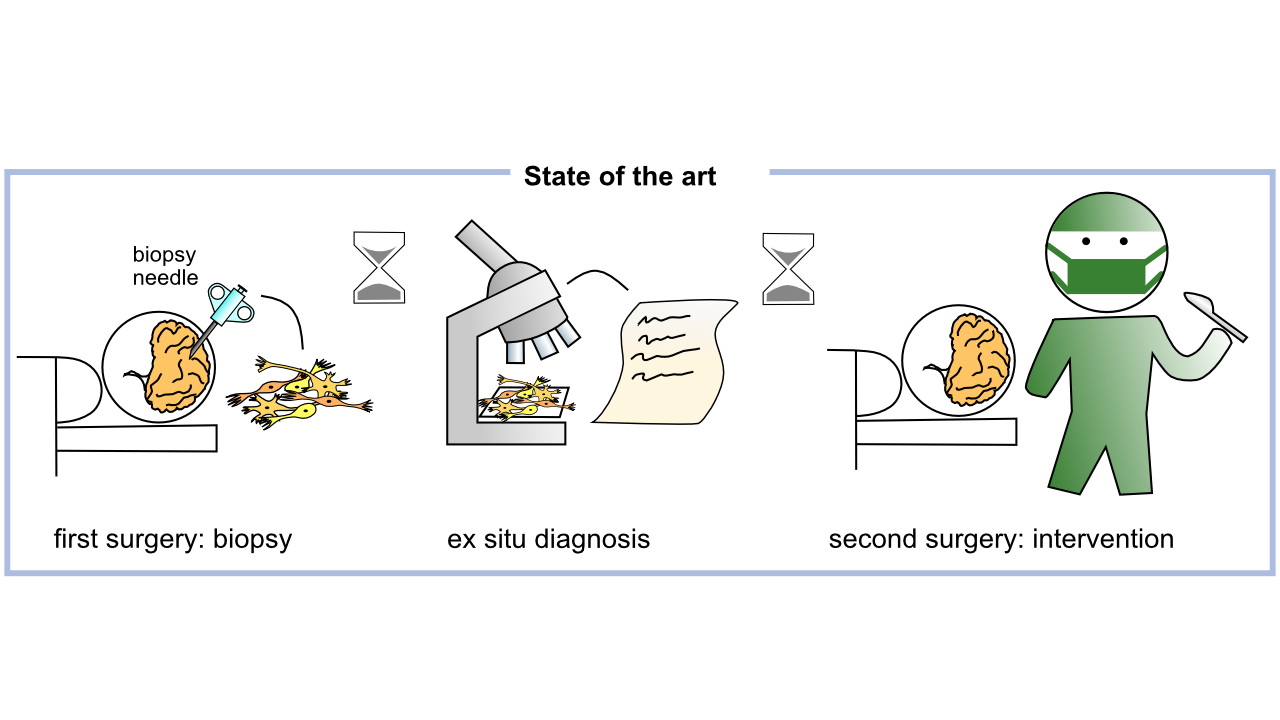
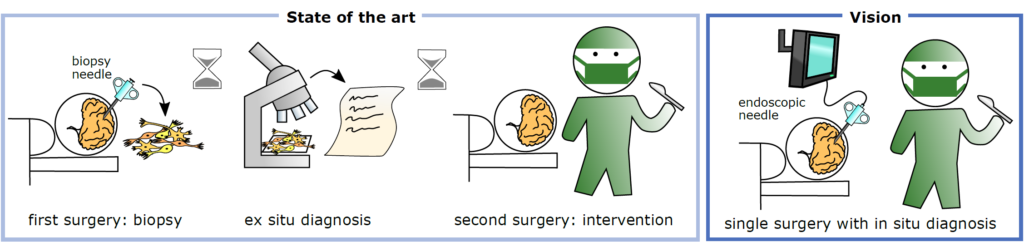
In patients with inoperable brain tumors located in eloquent brain regions, histological diagnosis is a prerequisite for the determination of the adjuvant treatment. This requires a minimal-invasive biopsy for histology that requires some days to provide an integrated diagnosis for further clinical decision-making. As a consequence, if the suspicious tissue turns out to be an aggressive brain tumor, the adequate therapy is delayed with negative impact on the patient’s prognosis. The development of strategies for direct tumor diagnosis bypassing tissue removal and lengthy pathological evaluation would allow the immediate therapy of affected patients.
We aim for intraoperative endoscopic brain tumor diagnosis, to eliminate the current time delay between stereotactic biopsies and diagnosis and to improve intervention results. Specifically, we will develop a miniaturized endoscope for auto fluorescence microscopy based on computational optics, which fits into biopsy needles. AI will be used to improve surgical decision making.
Endoscopy, in-vivo pathology, computational imaging, artificial intelligence
Kuschmierz, R., Scharf, E., Ortegón-González, D. F., Glosemeyer, T., Czarske, J., 2021. Ultra-thin 3D lensless fiber endoscopy using diffractive optical elements and deep neural networks. Light: Advanced Manufacturing.
Uckermann, O., Galli, R., Mark, G., Meinhardt, M., Koch, E., Schackert, G., Steiner, G., Kirsch, M., 2020. Label-free multiphoton imaging allows brain tumor recognition based on texture analysis-a study of 382 tumor patients. Neuro-Oncol. Adv. 2, vdaa035.
Galli, R., Meinhardt, M., Koch, E., Schackert, G., Steiner, G., Kirsch, M., Uckermann, O., 2019. Rapid Label-Free Analysis of Brain Tumor Biopsies by Near Infrared Raman and Fluorescence Spectroscopy-A Study of 209 Patients. Front. Oncol. 9, 1165.
Kuschmierz, R., Scharf, E., Koukourakis, N., Czarske, J.W., 2018. Self-calibration of lensless holographic endoscope using programmable guide stars. Opt. Lett. 43, 2997–3000.
Project team members |
Clinician |
TU Dresden, Faculty of Medicine, Carl Gustav Carus University Hospital Dresden
- PD Dr. med. habil. Witold Polanski, Department of Neurosurgery
- PD Dr. rer. nat. Ortrud Uckermann, Department of Neurosurgery and Division of Medical Biology, Department of Psychiatry and Psychotherapy
- Prof. Dr. med. Gabriele Schackert, Department of Neurosurgery
- Dr. med. Sven Richter, Department of Neurosurgery
- Katrin Kirsche, Departement of Neurosurgery
Project team members |
High-tech |
TU Dresden, Faculty of Electrical and Computer Engineering
- Dr.-Ing. Robert Kuschmierz, Chair of Measurement and Sensor Systems, Competence Center Biomedical Computational Laser Systems (BIOLAS)
- Prof. Dr.-Ing. Jürgen Czarske, Chair of Measurement and Sensor Systems, Competence Center Biomedical Computational Laser Systems (BIOLAS), coopted Professor Physics
- Dipl.-Ing. Jakob Dremel, Chair of Measurement and Sensor Systems
- Tijue Wang, Chair of Measurement and Sensor Systems
Abstract |
Primary brain tumors are neoplasms of the nervous system and the meninges and represent severe diseases due to the limited intracranial space. Therapies, prognosis and survival strongly depend on the tumor entity. For example, glioblastoma multiforme (GBM) is the most malignant type of brain tumor and affected patients have median overall survival times of less than two years despite therapy. GBMs represent ~50% of all primary brain tumors. To provide optimal therapy, accurate diagnosis is required as early as possible in the clinical workflow.
Pathological analysis requires some days to provide an integrated diagnosis that allows further clinical decision-making. If the suspicious tissue turns out to be an aggressive brain tumor, the adequate therapy (surgical removal, chemotherapy, radiotherapy) is, therefore, delayed (by days to weeks) with negative impact on the patient’s prognosis, especially in case of rapidly growing tumors. The development of strategies for direct tumor diagnosis bypassing tissue removal and lengthy pathological evaluation would allow the immediate therapy of affected patients.
We aim to develop and test a prototype of a novel tiny endoscope that probes autofluorescence of brain tissue and allows optical biopsies in situ. In the project, we will research the spectral characteristics of brain tumor fluorescence and miniaturize an endoscopic system while preserving high optical properties. This is achieved by implementation of recent advances in computational optics and programmable light. The development of tissue classification and strategies for integration of AI-supported diagnosis into the clinical workflow will allow successful translation. Moreover, the research may pave the way for future automated brain tumor diagnosis and tumor removal by laser ablation.

