Patient-specific devices for endovascular treatment of intracranial aneurysms
Medical Need |
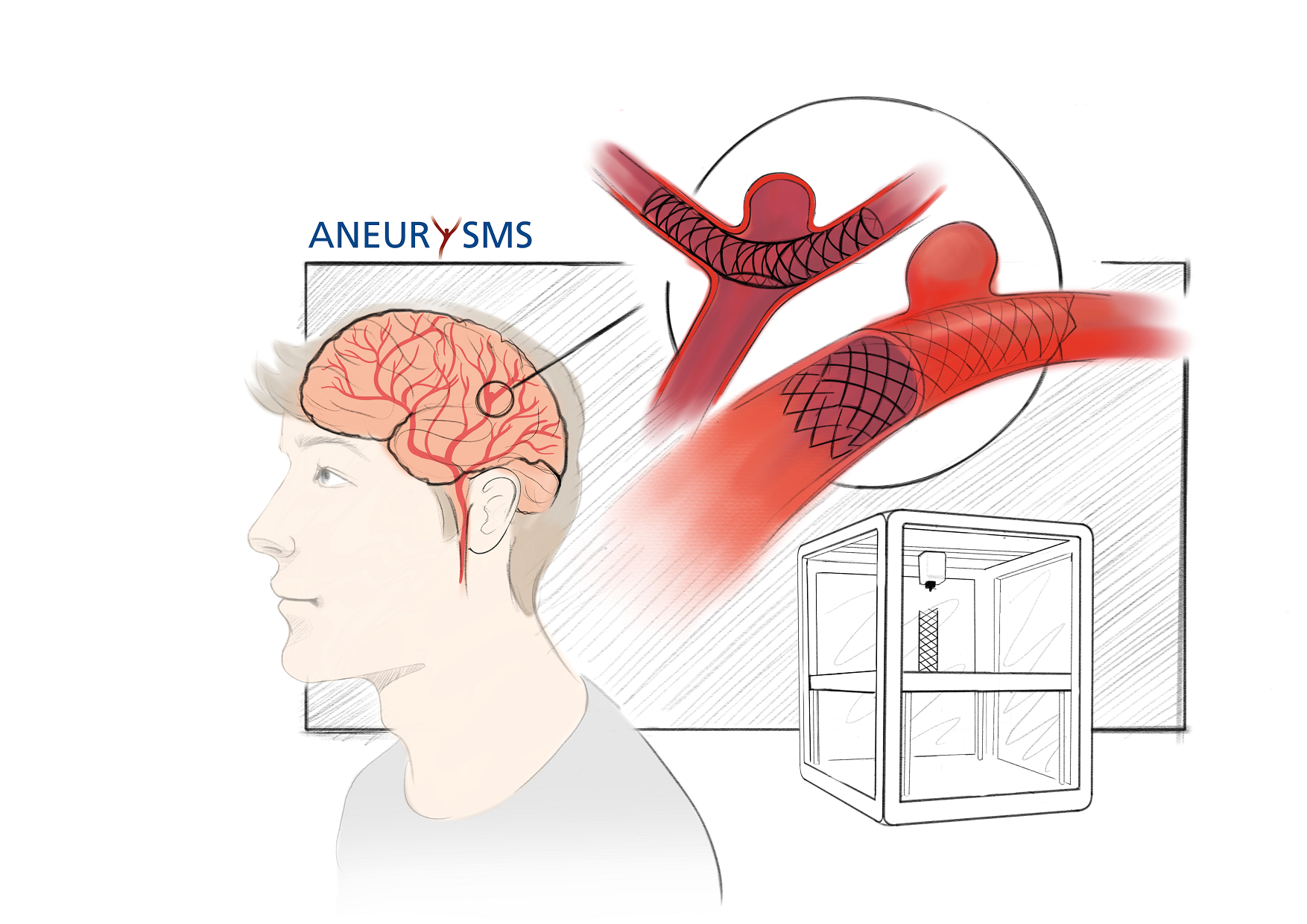
Intracranial aneurysms are treated in order to prevent their (re-)rupture. Today, endovascular treatment is often the preferred therapy approach. While the morphologies of aneurysms and their parent vessels vary greatly (Gawlitza et al., 2019), shapes of stents and intrasaccular devices are rather simple and uniform. Still, many aneurysms can only be treated by using devices not according to their actual indications (Gawlitza et al., 2018 & 2016). Additive manufacturing offers the possibility to produce patient-specific devices directly, thus making a high degree of individualization and optimal treatment possible.
The aim of the project is the development of a complete process chain for designing and manufacturing patient-specific devices for the treatment of intracranial aneurysms.
Patient-specific device, additive manufacturing, 3D-printing, intracranial aneurysm, nickel-titanium alloy, Shape-Memory-Alloy, digital process chain, endovascular treatment, personalized medicine
Korn, H., Koch, P., Kordaß, R., Holtzhausen, S., Schöne, C., Müller, B., Stelzer, R., 2018. Adapted scan strategy and slicing tool for improvement of strut precision in lattice structures. 2018 ASPE and euspen Summer Topical Meeting Volume 69. Berkeley, USA (22-25 July 2018), 50-54.
Gawlitza, M., Januel, A.-C., Tall, P., Bonneville, F., Cognard, C., 2016. Flow diversion treatment of complex bifurcation aneurysms beyond the circle of Willis: a single-center series with special emphasis on covered cortical branches and perforating arteries. J. NeuroInterventional Surg. 8, 481-487.
Gawlitza, M., Soize, S., Januel, A.-C., Mihalea, C., Metaxas, G.-E., Cognard, C., Pierot, L., 2018. Treatment of recurrent aneurysms using the Woven EndoBridge (WEB): anatomical and clinical results. J. Neurointerventional Surg. 2019 [Epub ahead of print]
Gawlitza, M., Soize, S., Manceau, P.-F., Pierot, L., 2019. An update on intrasaccular flow disruption for the treatment of intracranial aneurysms. Expert Rev. Med. Devices 16, 229–236.
Gustmann, T., Gutmann, F., Wenz, F. et al., 2020. Properties of a superelastic NiTi shape memory alloy using laser powder bed fusion and adaptive scanning strategies. Prog Addit Manuf 5, 11–18.
Grunert, R., Wagner, M., Preßler, N., Funke, K., Rotsch, C., Essig, H., Posern, S., Pabst, F., Drossel, W.-G., Lichtenstein, J., 2017. Concept of patient-specific shape memory implants for the treatment of orbital floor fractures. In: Oral and maxillofacial surgery 21. Nr.2, S.179-185.
Hermann, D.M., Steiner, T., Diener, H.-C. (Eds.), 2010. Vaskuläre Neurologie, 1st ed. Thieme
Abstract |
Intracranial aneurysms are frequently treated endovascularly using coils, stents and intrasaccular devices. However, aneurysms and their parent vessel are highly variable structures and although a broad armamentarium exists, many of these aneurysms can only be treated by using devices not according to their indications. While patient-specific manufacturing of vascular prostheses in the treatment of aortic aneurysms has been intensively investigated, an attempt in this direction has never been made in endovascular intracranial aneurysm treatment.
In the present project we explore possible applications of patient-specific devices made of superelastic nickel-titanium alloy. We aim to define aneurysm classes in which such personalized therapy approaches may be beneficial. A holistic digital process chain leads to a patient-specific device design, which will then be fabricated by a novel production method in this field called additive manufacturing (also known as 3D-Printing) utilizing laser beam melting (PBF-LB/M). The prototype is tested on an individualized, additively manufactured aneurysm model. Furthermore, the establishment of an animal aneurysm model is envisaged.

