Virtual companions, real responsibility
Living Labs in Medicine
In a new publication in the journal “Science Advances”, researchers from the Else Kröner Fresenius Center (EKFZ) for Digital Health at TUD Dresden University of Technology, in collaboration with the Center of Clinical Neuroscience at Dresden University Hospital and members of the TUD ethics committee, present a roadmap for implementing safe, regulation-compliant “Living Labs” in medicine. The authors emphasize the importance of flexible and safe frameworks that accelerate innovation in digital, AI-enabled healthcare.
Stephen Gilbert, Rebecca Mathias, Anett Schönfelder, Magdalena Wekenborg, Julia Steinigen-Fuchs, Anja Dillenseger, Tjalf Ziemssen: A roadmap for safe regulation-compliant Living Labs for AI and digital health development. Science Advances, 2025.
Living Labs: co-creative, near real-world environments for developing and testing innovations
Living Labs in medicine reflect real-world, collaborative environments designed to test new technologies in near-clinical conditions. Modern digital devices are increasingly flexible, connected and interdependent. Living Labs provide a valuable opportunity to study how patients or healthcare professionals interact with digital innovations – such as artificial intelligence (AI), mobile health apps and wearable sensors – in clinical workflows. By capturing user perspectives and outcomes at an early stage of development, living labs help to ensure that digital applications are both practical and have added value for patients. However, the flexibility that characterizes Living Labs often conflicts with the rigid structures of current EU regulatory frameworks for medical devices – frameworks that were not originally designed for digital and adaptive technologies.
Rebecca Mathias
Living Labs exist at a natural tension point with regulation. However, flexibility and safety are not mutually exclusive. Flexibility in evaluation and interaction with patients goes hand in hand with safety and ethical guardrails.”


The publication draws on the team’s experience establishing a university hospital-based Living Lab focused on Multiple Sclerosis (MS) research.
Tjalf Ziemssen
Our MS Living Lab is an evolving concept that is shaped through continuous engagement with all stakeholders, including the ethics committee and regulatory bodies, to maintain safety and compliance without hindering innovation”, says Prof. Tjalf Ziemssen, Head of the Center of Clinical Neuroscience at the Department of Neurology at Dresden University Hospital Carl Gustav Carus.
Digital Health Technologies require new evaluation approaches
The publication outlines how Living Labs – particularly those embedded in university medical centers – can comply with essential safety and ethical standards, while promoting digital innovation. The authors argue that environments such as Living Labs and “Regulatory Sandboxes” – which bring together developers and regulators to safely test emerging technologies – are essential for future healthcare technologies. The EU AI Act mandates the establishment of at least one Regulatory Sandbox per member state by August 2026, underlining the urgency of developing structured, ethical, and scalable experimentation spaces.
Unlike conventional medical devices, AI-enabled digital health technologies evolve over time and often require ongoing adjustments based on new data. This dynamic makes it difficult for developers to assess their tools in fully realistic clinical workflows, especially under current regulatory constraints. As a result, important aspects such as user experience, integration into existing hospital systems, and interaction with other devices are often overlooked. By providing a secure framework for ethical and legally compliant studies, Living Labs can help overcome these challenges. They support the collection of rich, real-world data and help developers better understand the needs of patients and healthcare professionals – ultimately accelerating the safe translation of innovative ideas into clinical practice.

Stephen Gilbert
The EU has outlined an ambitious regulatory vision to lead the way in the safe and ethical oversight of AI in medicine. This requires bold, well-funded strategies that support controlled studies and encourage innovation. With the right support, environments like Living Labs can bridge the gap between regulation and innovation – ensuring that digital health solutions evolve in ways that are meaningful, ethical, and ultimately beneficial to patients and healthcare systems.
More News
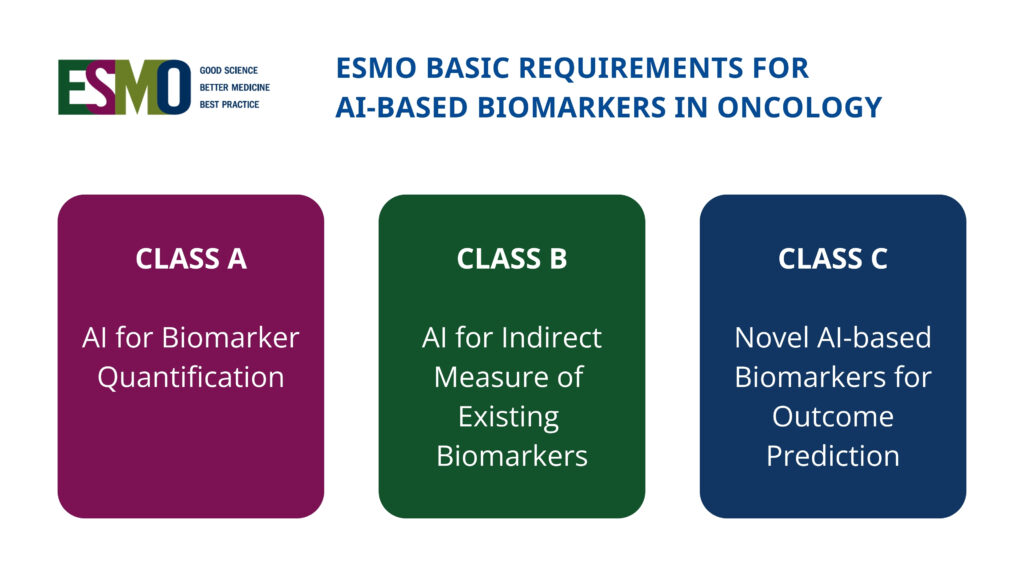
New international framework defines standards for AI-based biomarkers in oncology (EBAI)
New ESMO Guidelines: Safe use of large language models in oncology