Virtual companions, real responsibility
Digital Health Applications
In a paper published today in Nature Medicine, researchers show how user trust and pathways to market for digital health applications could be enhanced. They propose integrating transparent and mandatory feedback collection mechanisms directly into user interfaces. The approach is simple – similar to app store ratings – with feedback linked to a national platform. This could significantly improve user experience, increase patient safety by early identification of problems, and open-up faster approvals, reduce administration in monitoring tools and enable trusted digital health transformation.
Rebecca Mathias, Baptiste Vasey, Anastasia Chalkidou, Lars Riedemann, Tom Melvin, Stephen Gilbert: Safe AI-enabled digital health technologies need built-in open feedback, Nature Medicine, 2025. doi: 10.1038/s41591-024-03397-6.
AI-enabled digital health technologies (DHTs) are transforming healthcare, supporting diagnosis, therapy and lifestyle adaptation. However, the lack of real-time feedback on safety and performance limits their reliability. The authors from Else Kröner Fresenius Center (EKFZ) for Digital Health at TUD Dresden University of Technology together with colleagues explain that in the past regulations were often developed after medical failures. Examples being the Thalidomide tragedy in the 1960’s or defective breast implants, that were one of the factors leading to the implementation of the European Union Medical Device Regulation (EU MDR).
The current approval process for medical devices varies widely, in contrast to the very strict regulations for pharmaceuticals. There is an urgent need for robust post-market feedback systems for DHTs to gather real-world evidence and identify safety issues as early as possible. Although some feedback mechanisms already exist, there is a lack of international harmonization and integrated tools that allow for immediate and open user feedback. Unlike traditional medical devices such as implants, digital tools could readily include direct feedback approaches facilitating greater trust and use.
The research group studies Medical Device Regulatory Science and is among very few in Europe that focusses their research on adapting innovative regulatory practices. In a series of publications, the team has proposed strategies to enhance transparency, feedback, accountability, performance, and robust post-market monitoring of DHTs.

Rebecca Mathias
“Our work at the intersection of medical device regulations and digital health focuses on balancing innovation with safety. As digitization accelerates, we emphasize the need to develop fit-for-purpose regulations while leveraging existing frameworks to ensure the effective and safe implementation of digital technologies – recognizing that regulatory updates take time”, says Rebecca Mathias, lead author of the paper and researcher in the team of Professor Stephen Gilbert at EKFZ for Digital Health.

Stephen Gilbert
“Our research outlines standardized, system-wide surveillance frameworks to monitor the real-world performance of DHTs, including AI-enabled solutions with built-in mechanisms to foster safety, trust, and adaptive governance. Together, this research promotes a cycle of continuous improvement driven by stakeholder accountability, stronger regulatory compliance, user-centric innovation, and more efficient approaches to monitoring. These enable confidence in faster approvals, drive better uptake in health systems and, in through this, greater healthcare system efficiency”, explains Professor Stephen Gilbert.
Related Articles:
Welzel, C, Brückner, S, Brightwell, C, Fenech, M & Gilbert, S: A transparent and standardized performance measurement platform is needed for on-prescription digital health apps to enable ongoing performance monitoring. PLOS Digital Health (2024). doi: https://doi.org/10.1371/journal.pdig.0000656
Welzel C, Cotte F, Wekenborg M, Vasey B, McCulloch P, Gilbert S: Holistic Human-Serving Digitization of Health Care Needs Integrated Automated System-Level Assessment Tools. J Med Internet Res (2023) doi: 10.2196/50158
Gilbert, S, Pimenta, A, Stratton-Powell, A, Welzel, C, & Melvin, T: Continuous Improvement of Digital Health Applications Linked to Real-World Performance Monitoring: Safe Moving Targets? Mayo Clinic Proceedings: Digital Health (2023). doi: https://doi.org/10.1016/j.mcpdig.2023.05.010
Gilbert S, Anderson S, Daumer M, Li P, Melvin T, Williams R: Learning From Experience and Finding the Right Balance in the Governance of Artificial Intelligence and Digital Health Technologies. J Med Internet Res (2023) doi: 10.2196/43682
This work was supported by the European Commission under the Horizon Europe Program, as part of the project ASSESS-DHT (101137347). UK participants in Horizon Europe Project ASSESS DHT are supported by UKRI grant number 10106825 (National Institute for Health and Care Excellence) and 10108522 (University of Oxford).
More News
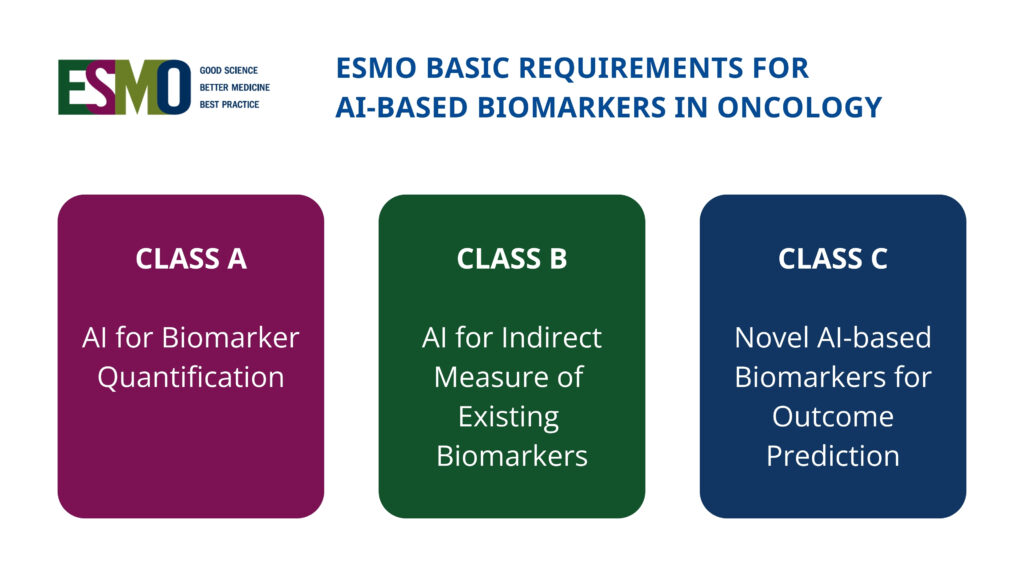
New international framework defines standards for AI-based biomarkers in oncology (EBAI)
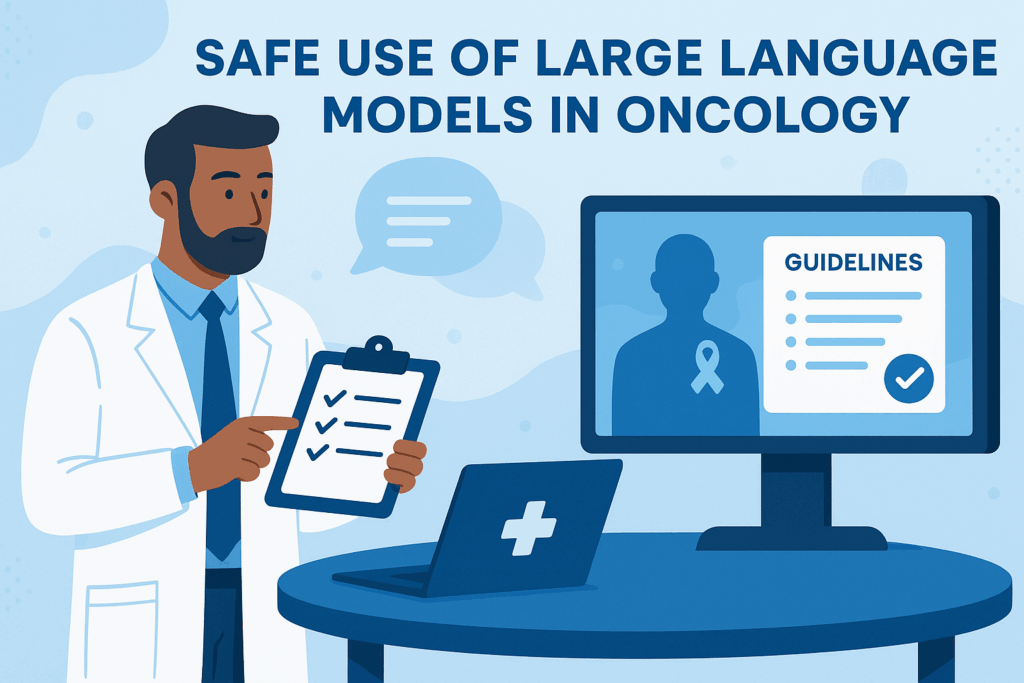
New ESMO Guidelines: Safe use of large language models in oncology