Virtual companions, real responsibility
New international framework defines standards for AI-based biomarkers in oncology (EBAI)
ESMO, the European Society for Medical Oncology, has released a landmark publication, which outlines the minimum requirements for the validation and clinical use of artificial intelligence (AI)-based biomarkers in oncology. Developed through a rigorous, multi-round consensus process, the ESMO Basic requirements for AI-based biomarkers in oncology (EBAI) framework represents the first comprehensive guidance for the safe, trustworthy and effective integration of AI-derived biomarkers into cancer care.
ESMO Basic Requirements for AI-based Biomarkers In Oncology (EBAI)
DOI: 10.1016/j.annonc.2025.11.009
Prof. Jakob N. Kather
Deputy Chair of the ESMO Real World Data & Digital Health Task Force, co-author of the publication
AI systems can function as biomarkers because they are able to analyse complex, multidimensional data to predict disease features and clinical outcomes, including treatment responses in patients with cancer. These AI systems process information and identify patterns that may even be imperceptible to humans, effectively transforming data into actionable clinical insights. As AI technologies increasingly permeate oncology—from pathology and imaging to genomics and electronic health records—there is a growing urgency to ensure that AI-based biomarkers meet robust validation criteria before being used to inform treatment decisions. While there are hundreds of papers and dozens of products using AI to measure biomarkers, there has been no conceptual framework, no guidance on how to validate and use them. That’s precisely what EBAI aims to provide.
Dr. Benedikt Westphalen
Chair of the ESMO Precision Oncology Task Force, co-lead author of the publication
The EBAI framework addresses this need by offering a clear, actionable guidance for developers, clinicians, regulators and healthcare institutions. EBAI creates shared language and consistent benchmarks to guide all parties involved. This guidance will help developers understand evidence requirements before investing in extensive validation studies, assist clinicians in determining when an AI-based biomarker is sufficiently validated for clinical use, and support regulatory bodies and healthcare institutions in setting appropriate standards for implementation. We have entered a new era where AI tools are not just supporting clinicians: they are becoming surrogate biomarkers. That’s a major shift, and it demands clear rules. EBAI was born out of the need to define what ‘validated’ means in this context.
A New Classification System for AI Biomarkers
The EBAI framework introduces a classification system that addresses the diversity of AI biomarkers in oncology:
- Class A: AI that quantifies existing biomarkers using the same input data as standard biomarkers/assays.
- Class B: AI systems that act as indirect measures of known biomarkers using AI-based alternative methods, often used for screening.
- Class C: Novel AI-derived biomarkers trained directly on clinical outcomes, subdivided into C1 (prognostic) and C2 (predictive) categories.
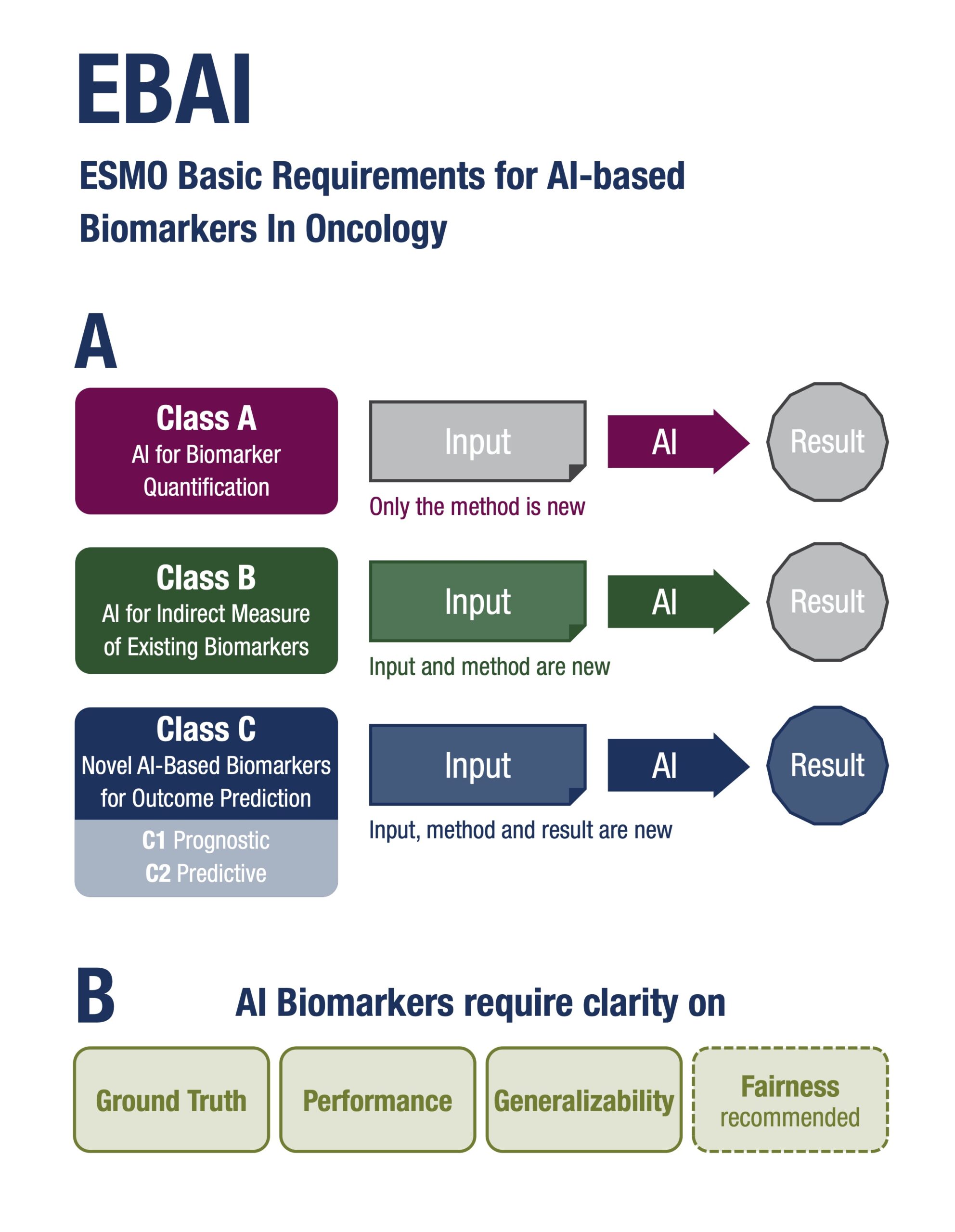
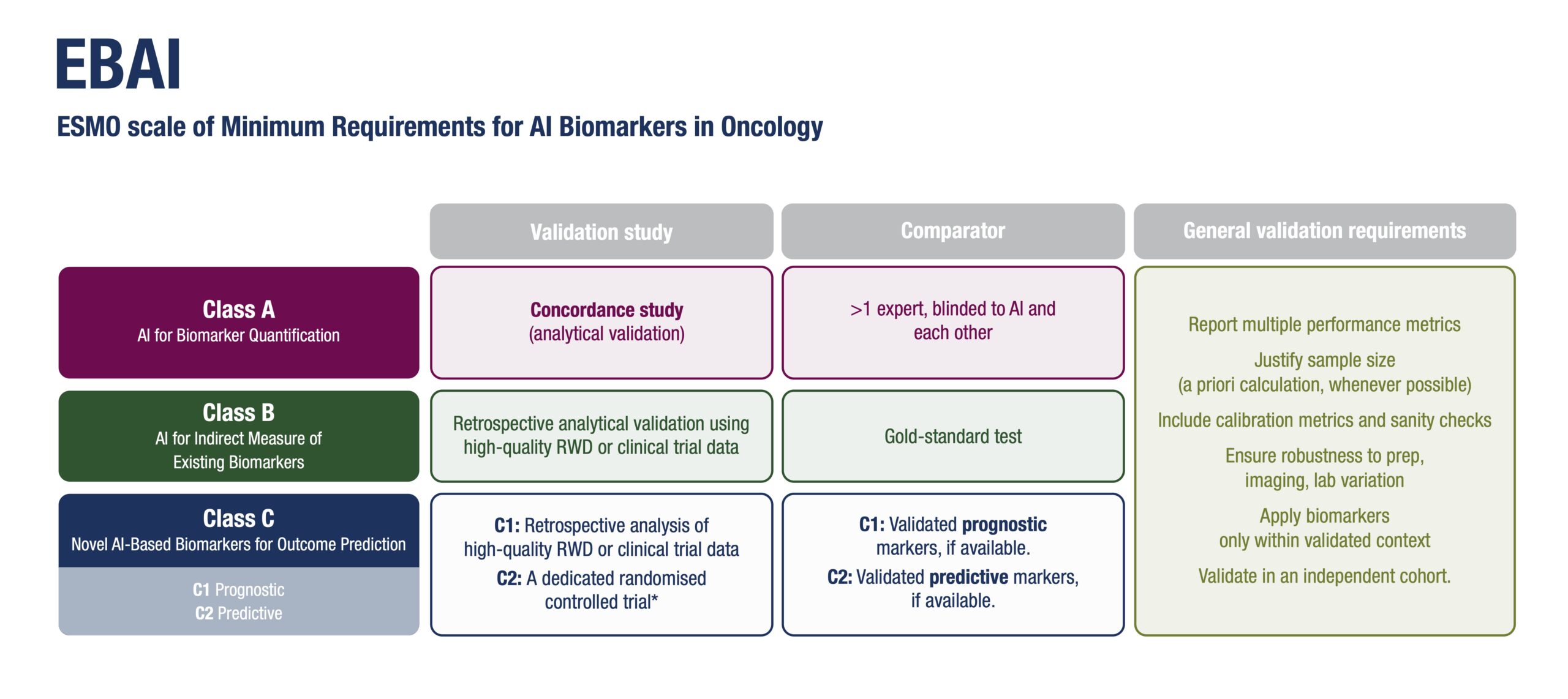
Each class is associated with tailored validation requirements, ranging from concordance studies to retrospective and prospective clinical trials, depending on the intended clinical use and risk level. Class B biomarkers could be transformative. “Imagine screening hundreds of thousands of patients using AI on histology slides, then confirming only the positives with molecular tests. That’s a scalable, cost-effective approach, that could be applied and have a global impact,” observed Mihaela Aldea, first author of the paper, Institute Gustave Roussy, Villejuif, France.
Consensus-Driven Standards for Clinical Adoption
The guidance was developed by a multidisciplinary panel of 37 international experts, including oncologists, pathologists, radiologists, computational scientists, biostatisticians, ethicists, a patient advocate, regulatory experts. Using a four-round voting methodology, the panel reached consensus on key dimensions for evaluating AI-based biomarkers, including: Ground truth and comparator standards; Performance metrics and calibration; Generalisability across populations and technical settings; Fairness and bias mitigation; Explainability and interpretability; Cost-effectiveness and turnaround time.
Ground truth, performance, and generalisability are non-negotiable: “An AI biomarker needs to show consistent performance across diverse settings and populations before it can be considered for clinical use,” said Aldea. “Explainability isn’t just a technical detail—it’s a safeguard,” she continued. Sanity checks and interpretability experiments help ensure that AI isn’t relying on spurious patterns or shortcuts. “For oncologists to use AI-based biomarkers, we need robust clinical validation showing a clear, measurable benefit and no harm for our patients,” she stressed. “It’s about finding the right balance—avoiding mistrust that delays progress but also over-reliance without reflection. For that, respecting quality standards is essential.” The framework also recommends confirmation in multiple datasets, post-deployment monitoring and encourages prospective testing in real-world workflows to build trust and ensure sustained performance.
Reinforcing ESMO’s Role in Shaping the Future of Oncology
With EBAI, ESMO continues to lead the conversation on the responsible integration of digital technologies in cancer care. This publication builds on previous efforts, including guidance on the use of large language models in oncology (ELCAP), and aligns with ESMO’s broader commitment to the implementation of precision oncology and the meaningful use of real-world data. “ESMO is the first scientific professional society to take an official position on AI-based biomarkers. EBAI is a starting point, at the basis of a long-lasting commitment bringing together developers, clinicians and regulators to improve the life of patients with cancer,” said ESMO President, Fabrice André.
The full publication is available here and will serve as a reference for future regulatory discussions, clinical implementation strategies, and industry development efforts. References: DOI: 10.1016/j.annonc.2025.11.009 – URL: https://www.annalsofoncology.org/article/ S0923-7534(25)06267-2/fulltext
More News
New ESMO Guidelines: Safe use of large language models in oncology