Virtual companions, real responsibility
Digital medical care
at home
Recently, the US Food and Drug Administration (FDA) has announced an ambitious initiative that will explore solutions for equitable health care at home. Researchers from the EKFZ for Digital Health and TUD Dresden University of Technology now share their views on the initiative as well as the opportunities and challenges of establishing digital health solutions in home environments. Their thoughts have been published in the Nature Portfolio journal npj Digital Medicine.
Stefanie Brückner, Celia Brightwell, Stephen Gilbert: FDA launches health care at home initiative to drive equity in digital medical care; npj Digital Medicine, 2024.
Stephen Gilbert, Professor of Medical Device Regulatory Science, together with researchers Stefanie Brückner and Celia Brightwell has analyzed the recent US efforts that aim at improving digital medical care and facilitating the adoption of new digital health concepts.
Challenges in digital health technology development
Despite the increase in digital health applications during the COVID-19 pandemic, many of which continue to this day, implementation of new technologies is still relatively slow. There is evidence that digital health technologies are not being developed coherently and holistically to enable useful, sustainable, ergonomic, and transformative integration into patients’ lives and environments. Current product development and regulations result in digital health tools being developed and operating in islands rather than facilitating integrated digital care. Patients have to use and combine tools that were designed as isolated products, many of which were never intended for use in the home environment. Current regulatory and approval strategies continue to focus on isolated new products rather than flexible device suites.
Need for system-level regulatory thinking
The researchers welcome the recent ambition of three US federal administrations responsible for health regulation. Now, there is a growing recognition that the home environment can and will be an integral part of the health care system in the future. However, the authors emphasize that such home-based devices and platforms must meet the highest cybersecurity standards and require approval and reimbursement strategies for flexible device suites.
Enhancing health care equity
Care-at-home solutions offer an opportunity to address health care inequities and could help ensure health care delivery for millions of people with limited access to the health care system. The researchers acknowledge the ambitious FDA initiative and its willingness to address these challenges. They emphasize the need for health regulation and technology assessment to consider cross-product evaluation approaches, in addition to the flexible design of digital product suites.
More News
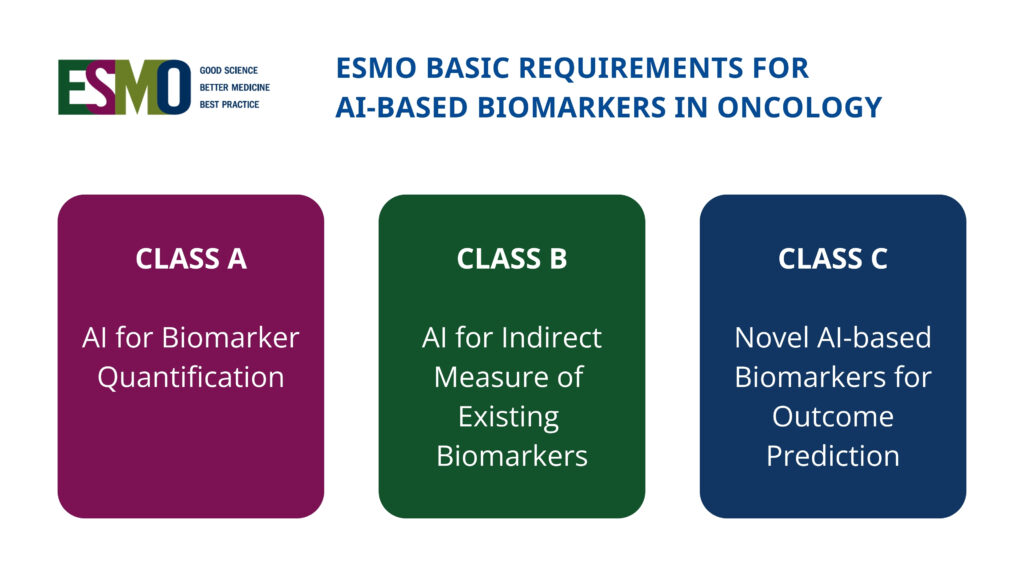
New international framework defines standards for AI-based biomarkers in oncology (EBAI)
New ESMO Guidelines: Safe use of large language models in oncology